The reaction between molecular hydrogen (H2) and another compound or element to reduce or saturate the organic compound under the action of a catalyst (nickel, palladium or platinum) or at a very high temperature is called a hydrogenation reaction. Hydrogenation reduces the double and triple bonds in the hydrocarbon.
Catalysts:
Hydrogenated catalysts generally fall into two broad categories: homogeneous catalysts and heterogeneous catalysts. The homogeneous catalyst can be dissolved in a solvent containing an unsaturated substrate. The non-homogeneous catalyst refers to a solid catalyst which is also suspended in the same solvent as the reaction or treated with a gaseous substrate. As depicted in figure 1 are several common homogeneous hydrogenation catalysts.
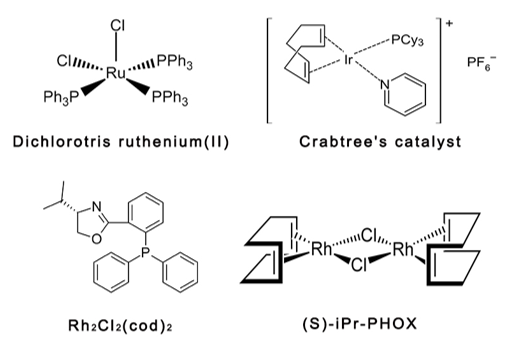 Figure 1. The schematic diagram of several common homogeneous hydrogenation catalysts
Figure 1. The schematic diagram of several common homogeneous hydrogenation catalysts
More commonly found in the industry is a heterogeneous catalyst that is used for hydrogenation. Common hydrogenated heterogeneous catalysts are Raney nickel, Lindlar catalyst, Raney-Nickel catalyst, palladium-carbon, platinum, cobalt, and the like. In the industry, a precious metal hydrogenation catalyst is deposited as a fine powder from a solution on a support which is an inexpensive, bulky, porous, usually particulate material such as activated carbon, alumina, calcium carbonate or barium sulfate.
Applications:
The most common industrial hydrogenation catalyst is the heterogeneous catalyst mentioned above. Catalytic hydrogenation is mainly used in the food industry, petrochemical industry and organic chemistry. The following is a detailed introduction to the applications.
- Food industry: The largest scale application of hydrogenation is for the processing of vegetable oils. Typical vegetable oils are derived from polyunsaturated fatty acids (containing more than one carbon-carbon double bond). The degree of hydrogenation is controlled by limiting the amount of hydrogen, the reaction temperature and time, and the catalyst, which reduces most, but not all, of the carbon-carbon double bonds contained in the hydrogenation.
- Petrochemical industry: In petrochemical processes, hydrogenation is often used to convert olefins and aromatics to saturated alkanes (paraffins) and cycloalkanes, which has the advantage that the reaction is less toxic and less reactive and advantageous for storage. Because saturated hydrocarbons are more stable compared to alkynes and olefins, they exhibit superior storage properties. On the other hand, olefins tend to form hydroperoxides, which form gums, a substance that can be used to interfere with fuel processing equipment.
- Organic chemistry: In organic chemistry, hydrogenation is a useful measure to convert unsaturated compounds into saturated derivatives. The reaction substrate includes not only olefins and alkynes but also compounds containing an unsaturated bond such as an aldehyde, an imine and a nitrile. They are converted to the corresponding saturated compounds, namely alcohols and amines.
References
- Werkmeister, Svenja. (2014) 'Catalytic Hydrogenation of Carboxylic Acid Esters, Amides, and Nitriles with Homogeneous Catalysts'. Organic Process Research & Development. 18 (2): 289-302.
- Schrock, Richard R. (1976) 'Catalytic hydrogenation using cationic rhodium complexes. I. Evolution of the catalytic system and the hydrogenation of olefins'. Journal of the American Chemical Society. 98 (8): 2134-2143.
- Berkessel, Albrecht. (2002) 'Hydrogenation without a Transition-Metal Catalyst:On the Mechanism of the Base-Catalyzed Hydrogenation of Ketones'. Journal of the American Chemical Society. 124 (29): 8693-86938.
You Might Like Also

Magnetic Metal Complexes
Phosphorus Catalysts
Organic Solar Cells

Ligands for Functional Metal Complexes














